Abstract
Background: Treatment with Bruton tyrosine kinase (BTK) inhibitors has remarkably improved clinical outcomes for patients with chronic lymphocytic leukemia (CLL). Acalabrutinib is a highly selective, covalent BTK inhibitor approved for the treatment of CLL. Here we report the final analysis of the phase 1/2 study of acalabrutinib (multicenter, multicohort ACE-CL-001 study; NCT02029443) with median follow-up of 53 months in patients with relapsed/refractory (R/R) CLL/small lymphocytic lymphoma (SLL). Prior analyses were reported at median follow-up durations of 14 months (Byrd et al. N Engl J Med. 2016;374:323-32) and 41 months (Byrd et al. Blood. 2020;135:1204-13).
Methods: Adult patients with CLL or SLL who had received ≥1 prior treatment and had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2 were included in the R/R cohort of the study. In the dose-escalation portion of the study, patients were enrolled into dose cohorts at acalabrutinib dosages of 100, 175, 250, and 400 mg once daily (QD) and 100 mg or 200 mg twice daily (BID). In the phase 2 portion of the study, patients received oral acalabrutinib 100 mg BID or 200 mg QD, later switching to 100 mg BID, until progressive disease or unacceptable toxicity occurred. The primary study endpoint was safety. Secondary endpoints included overall response rate (ORR), duration of response (DOR), and progression-free-survival (PFS), with post hoc analysis of event-free survival (EFS). Response rates were based on the iwCLL 2008 criteria with modification for lymphocytosis.
Results: In total, 134 patients with R/R CLL/SLL received ≥1 dose of acalabrutinib (dosing information was previously reported; Byrd et al. Blood. 2020;135:1204-13). Median age was 66 years, and 97% of patients had ECOG PS ≤1, 73% unmutated IGHV, 27% del(17p), 20% del(11q), and 35% complex karyotype. The median number of prior therapies was 2 (range 1-13). At the final data cutoff date of July 15, 2021, at a median follow-up of 52.6 (range 0.56-88.8) months, 31% of patients remained on acalabrutinib. The most common reasons for discontinuing treatment were progressive disease (37%) and adverse events (AEs; 15%).
The most commonly reported AEs were consistent with those reported in the previous update (Table 1); no new AEs emerged with longer term follow-up. AEs leading to discontinuation that occurred in >1 patient were pneumonia (5 patients), anemia, neutropenia, diarrhea, and thrombocytopenia (2 patients each). Events of clinical interest (all grades; grade ≥3, respectively) included atrial fibrillation/flutter (9%; 3%), hypertension (23%; 11%), infection (88%; 33%), bleeding events (71%; 6%), major bleeding events (8%; 6%), and ventricular tachyarrhythmias (2%, 1%). No cases of sudden death were reported.
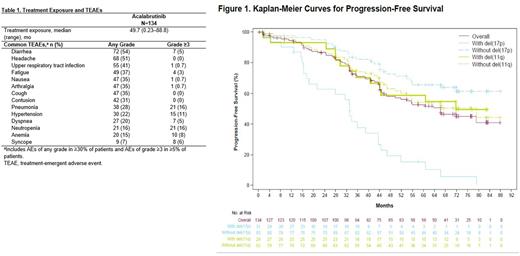
The ORR (partial response or better) was 90% (95% confidence interval [CI] 84-95); 4% of patients had complete response and 87% partial response (PR). The median DOR was 60.1 months (range 2.7-81.0). The 66-month estimated DOR rate was 45% (95% CI 36-55). Responses were similar regardless of high-risk features, including unmutated IGHV (n=73/81; 90%), del(17p) (n=27/31; 87%), del(11q) (n=20/23; 87%), and complex karyotype (n=17/20; 85%). The median PFS was 66.1 months (range 0.4-87.8; Figure 1), and the median EFS was 53.8 months (range 44.1-66.1). The 72-month estimated PFS was 45% (95% CI 36-54) and the 72-month estimated EFS was 37% (95% CI 29-46). Reported EFS events overall included AEs (n=18/134, 13%), death (n=5/134, 4%), disease progression (n=54/134, 40%), and new anticancer therapy (n=3/134, 2%).
Conclusions: The results of this final analysis confirm the earlier reports of acalabrutinib efficacy and were later confirmed by similar outcomes of response durability and long-term tolerability in the pivotal ASCEND phase 3 trial in patients with R/R CLL/SLL (Jurczak et al. J Clin Oncol. 2022;40[suppl 16]:7538). Reported AEs indicate a tolerable and consistent safety profile, with low rates of atrial fibrillation and major bleeding events with median follow-up of 53 months (up to 7.4 years). No new safety signals were identified.
Disclosures
Furman:AbbVie, AstraZeneca, Beigene, BMS, Genentech, Janssen, Loxo, MEI Pharma, Pharmacyclics, Sanofi, TG Therapeutics, X4 Pharmaceuticals: Consultancy. Wierda:Karyopharm: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Kite, a Gilead Company: Research Funding; Xencor: Research Funding; Genzyme: Consultancy; Sanofi: Consultancy; Juno: Research Funding; Sunesis: Research Funding; Pharmacyclics LLC: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Janssen: Research Funding; GSK/Novartis: Research Funding; Gilead Sciences: Research Funding; Genentech: Research Funding; Cyclacel: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; AbbVie: Research Funding. Schuh:AstraZeneca: Honoraria, Other: Non-educational grant; Janssen: Honoraria, Other: Non-educational grant; Gilead: Honoraria, Other: Personal fees; Roche: Honoraria, Other: Personal fees; SERENOx: Membership on an entity's Board of Directors or advisory committees, Other: Founder of; Abbvie: Honoraria, Other: Personal fees; Oxford Nanopore Technology: Other: In kind contributions; Illumina: Other: In-kind contributions; Adaptive Biotechnology: Honoraria; Exact Sciences: Honoraria. Patten:Roche: Research Funding; Gilead Sciences: Honoraria, Research Funding; Abbvie: Honoraria; AstraZeneca: Honoraria; Beigene: Honoraria; Janssen: Honoraria. Chaves:Epizyme: Honoraria; Ipsen: Honoraria. Brown:Abbvie, Acerta/Astra-Zeneca, BeiGene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, iOnctura, Janssen, MEI Pharma, Pharmacyclics: Consultancy; BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics: Research Funding. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Awan:Cellecter Bisosciences: Consultancy; Merck: Consultancy; BMS: Consultancy; Dava Oncology: Consultancy; Johnson and Johnson: Consultancy; BeiGene: Consultancy; Incyte: Consultancy; Verastem: Consultancy; MEI Pharma: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Kite Pharma: Consultancy; Gilead Sciences: Consultancy; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy; Cardinal Health: Consultancy; ADCT Therapeutics: Consultancy; Epizyme: Consultancy; Caribou Biosciences: Consultancy. Stephens:Mingsight: Research Funding; Novartis: Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy; Arqule: Research Funding; Acerta: Research Funding; Karyopharm: Research Funding; CSL Behring: Consultancy; Beigene: Consultancy; Celgene: Consultancy; Newave: Research Funding; TG Therapeutics: Consultancy; Epizyme: Consultancy; Lilly: Consultancy; Genentech: Consultancy; JUNO: Research Funding. Ghia:AstraZeneca: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Lilly/Loxo: Consultancy, Honoraria; Roche: Consultancy, Honoraria; MSD: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria. Barrientos:Beigene: Consultancy; AbbVie: Consultancy; Velosbio/Merck: Research Funding; AstraZeneca: Consultancy, Research Funding; Oncternal: Research Funding; Janssen: Honoraria; MEI: Consultancy; Pharmacyclics/Abbvie: Consultancy. Patel:Nurix: Research Funding; Caribou Biosciences: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; Trillium Therapuetics/Pfizer: Consultancy, Research Funding; Sunesis Pharmaceuticals: Research Funding; CRISPR Therapeutics: Research Funding; Xencor: Consultancy, Research Funding; Aptevo Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Epizyme: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; BeiGene: Consultancy; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Kite pharma: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; MEI Pharma: Consultancy, Research Funding; Curis, Inc: Research Funding; Morphosys: Consultancy; Fate Therapeutics: Research Funding; Genetech/Roche: Consultancy, Research Funding, Speakers Bureau; Adaptive Biotechnologies: Research Funding; ADC Therapeutics: Consultancy; Velos Bio: Research Funding. Woyach:Schrodinger: Research Funding; Genentech: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; MorphoSys: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pharmacyclics: Consultancy; Janssen: Consultancy; Newave: Consultancy; ArQule: Consultancy; Karyopharm Therapeutics: Research Funding; Loxo@Lilly: Research Funding. Butturini:AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. de Borja:AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company. Wang:AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. O'Brien:Acerta, Alliance, Beigene Ltd, Caribou Biosciences Inc, Gilead, Kite, Loxo Oncology, Mustang, Nurix Therapeutics Inc, Pfizer, Pharmacyclics, Regeneron, Sunesis, and TG Therapeutics.: Research Funding; AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, Bristol Myers Squibb, Celgene, DynaMed, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma Inc, Merck, NOVA Resea: Consultancy. Byrd:Ohio State University: Patents & Royalties; Kura: Consultancy; AstraZeneca: Consultancy; Newave: Consultancy; Trillium: Consultancy; Pharmacyclics: Research Funding; Zencor: Research Funding; Vincerx: Consultancy, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; AbbVie: Consultancy; Syndax: Consultancy; Janssen: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal